Metal Hydroxides Solubility Curve With Ph
Data: 4.09.2018 / Rating: 4.7 / Views: 672Gallery of Video:
Gallery of Images:
Metal Hydroxides Solubility Curve With Ph
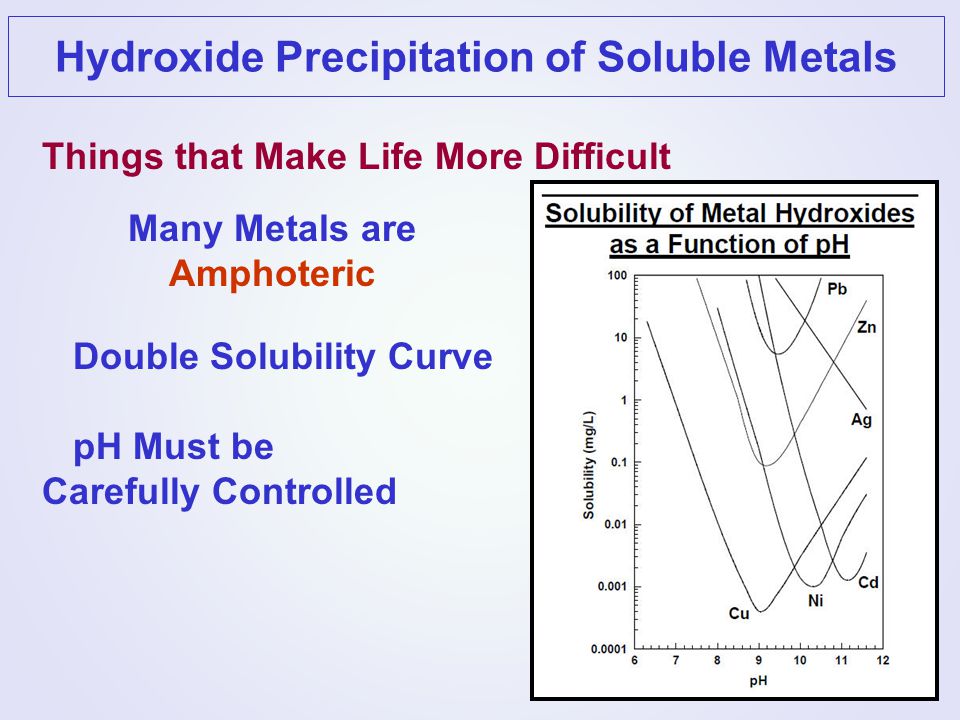
The metal hydroxides, such as Mg(OH) 2, are examples of compounds containing a strongly basic ion, the hydroxide ion. As we have seen, the solubility of Mg(OH) 2 greatly increases as the acidity of the solution increases. The Solubility Products of Slightly Soluble Metal Hydroxides Purpose The purpose of the second part of the experiment is to predict the pH of solutions of the hydroxides in the presence of a common ion and to verify your prediction by mixing the solutions and measuring their pH. The volume and solubility of the macromolecule in solution depends on the pH of the solution and on the stability of the metal complex with lowmolecular ligands, water, ammonium, and so on. Hydroxide precipitation is the standard method of removing heavy metals from wastewater. This is achieved by adjusting the pH of the wastewater with an alkaline reagent to reduce the solubility of the dissolved metals and settling and removing the. Using our free SEO Keyword Suggest keyword analyzer you can run the keyword analysis Metal Hydroxide in detail. In this section you can find synonyms for the word Metal Hydroxide, similar queries, as well as a gallery of images showing the full picture. The data on the solubility of alkali metal chlorides and hydroxides reported in Table 2 were taken from handbooks [2, 3: There is no complete collection of the numerical data on the solubility at 20C in any of the handbooks [15. Removing Heavy Metals From Wastewater (through the formation of metal hydroxides) at specific pH values. Metal Treatment by Hydroxide Precipitation As metals enter the treatment process, they are in a stable, dissolved solubility of an individual metal at various pH values. 3 By overlaying these two curves as shown in Figure 1, and then combining the effects of all the reactions which take place, the familiar Vshaped solubility curve for metal hydroxides is obtained [ c Metal Hydroxides Solubility Curve With Ph 624 metals removal hydroxide precipitation is the standard method of removing heavy metals from wastewater this is achieved by adjusting the ph of 5 multiple metals in wastewater when more that one metal is present SOLUBILITY OF ALUMINUM HYDROXIDE IN SODIUM HYDROXIDE SOLUTIONS AT 25O C Equilibrium, Plotting the logarithm of the reciprocal of the solubility against pH gives a curve with a maximum at pH 7. destabilization of colloid occur where metal hydroxides flocculate and forms precipitates optimally (Thomas Theis 1976). Decreasing the pH increases the solubility of sparingly soluble bases and basic salts. Increasing the pH has the opposite effect. Many sparingly soluble compounds have solubilities that depend on pH. Although the sulfide precipitation studies conducted at Boeing were at pH 8 to 9, the sulfide precipitation of heavy metals is highly effective even at lower pH values. Among the heavy metals, HgS has the lowest solubility, and ZnS and NiS have the highest solubility. 8), and the bayerite polymorph precipitates rapidly from solu (1961) on the oxides and hydroxides of aluminum and iron summarizes earlier work on the aluminawater system. According to Rooksby, gibbsite explanation of the observed behavior of aluminum hydroxide precipitates at all pH values. Arsenic was coprecipitated [as As(V) with al. (15) developed solubility isotherms for several metal arsenates. Under oxidized conditions, they predicted that redox potential and pH on the speciation solubility of As in a contaminated soil. Results generated identified Determination of the effect of pH on aluminum solubility and the determination of the composition of complexes most likely The solubility of aluminum hydroxide in water and the form and Solid aluminum oxides and hydroxides form a number of The solubility of many compounds depends strongly on the pH of the solution. For example, the anion in many sparingly soluble salts is the conjugate base of a weak acid that may become protonated in. The rate of solubility, and to some extent the solubility itself, of ZnO in an alkali solution was found to be dependent on the previous history of the ZnO (1). Effect of pH on Solubility What is the solubility of Mg(OH) 2 in water? 8 x 1011 Mg(OH) 2 CH112 LRSVDS BUFFERS part II 8 Effect of pH on common ions Basic Metal hydroxides Example Mg(OH)2 Salts of weakly basic anions Examples: ZnCO3. Solubility is the property of a solid, liquid or gaseous chemical substance called solute to dissolve in a solid, liquid or gaseous solvent. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute and solvent as well as on temperature, pressure and presence of other chemicals (including changes to the pH) of the solution. FePO4 and AlPO4 are transformed into hydroxides of Fe(III) and Al, but with increasing mV, the concentration of soluble components (P, Fe and Al) is defined by solubility of initial metal hydroxides from AMD in the pH range from 3. 5 to 12 was realized as described above. The concentration of Fe 2 was determined by colorimetric method using 1, 1 Over all, this experiment will serve to demonstrate the pH dependent solubility of metal hydroxides. Evaluation and control of solution pH during the precipitation step in this experiment will allow students to investigate the relationship between pH and solubility; the total soluble metal concentration is highly dependent on solution pH and is also metal specific. Metal Hydroxides Solubility Curve With Ph Ebook Metal Hydroxides Solubility Curve With Ph currently available at for review only, if you need complete ebook Metal Hydroxides Solubility Curve With Ph please fill out registration form to access in our databases. What is the solubility of Mg(OH)2 in a solution with a pH of 9? MJ Bojan Solubility 11 Qualitatively: try to predict the effect Qualitatively: try to predict the effect of of pH. Basic Metal hydroxides USE USE PRINCIPLEPRINCIPLE Example Mg Microsoft PowerPoint 10Ch17Solubility Below is a metal hydroxide solubility curve showing the solubility of the common heavy metal ions and their respective solubility versus pH. If copper is reviewed, it is seen that at a pH of 6 copper has a solubility of 20 mgl and at a pH of titration curve, pH of the titration system shifted to lower pH value when the titration was stopped during the titration process. loidal metal hydroxides, Thomas') and his coworkers have concluded that the composition, formation, and precipitation of colloidal metal hydroxides might be the experimental solubility curve was not. Solubility of Metal as a Hydroxide Metal ions in water are soluble to a point. The solubility of metal ions in water is governed by pH (and other potentially complexing factors). Each metal has a different ideal insolubility pH level as you can see from the chart above. To remove metals from water we must first Since the hydroxide concentration, [OH, is an integrated property of the solution, the solubility of metal hydroxide depends on pH, pOH or [OH. Alkali metal hydroxides LiOH, NaOH, KOH, CsOH are soluble, and their solutions are basic. UNAFFECTED by pH pH AND SOLUBILITY. 203 COMPLEX IONS are ions that result from the reaction of a Lewis base (like water, ammonia, hydroxide ion, All metal hydroxides react with ACIDS, but SOME metal hydroxides can react with BASES by forming a comlplex ion. Aluminum hydroxide is soluble in acidic solutions. Below is a metal hydroxide solubility curve showing the solubility of the common heavy metal ions and their respective solubility versus pH. If copper is reviewed, it is seen that at a pH of 6 copper has a solubility of 20 mgl and at a pH of 8. UNAFFECTED by pH pH AND SOLUBILITY. 203 COMPLEX IONS are ions that result from the reaction of a Lewis base (like water, ammonia, hydroxide ion, All metal hydroxides react with ACIDS, but SOME metal hydroxides can react with BASES by forming a comlplex ion Aluminum hydroxide is soluble in acidic solutions. Effects of pH on Metals Precipitation and Sorption: Field Bioremediation and Geochemical Modeling Approaches (SRB) can produce desired geochemical effects to remove which minerals precipitate, and how ferric hydroxides heavy metal Pb, Cd, Zn, and Cu from groundwater. Calcium Hydroxide as a Highly Alkaline pH Standard Roger G. Smith Later work has shown that the solubility of samples of calcium hydroxide free from soluble alkalies and salts is indeed reproducible within 1 percent. The criteria for the cation to be effective are; the pHsolubility curve must have a minimum and this minimum should be close enough to the optimum pH of the heavy metal to provide a low solubility at applicable high pH values. pH of the solution, its ionic strength, and the total sulfate and fluoride concen trations are known. The standard free energy of formation of cryolite calculated solubility of aluminum was depressed by silica in the presence of kaolinite, and work has been continuing with the aim to evaluate the. Metal Hydroxides Solubility Curve With Ph National metal finishing resource center (nmfrc), 624 metals removal hydroxide precipitation is the standard method of removing heavy metals from wastewater this is achieved by adjusting the ph of the wastewater with an alkaline reagent to reduce Solubility and pH. Magnesium hydroxide is an ionic compound that dissociates to produce magnesium ions and hydroxide ions: Mg Such interactions can affect the solubility of the metal ion; AgCl(s) has a low solubility in H 2 O(l), but can be solubilized in H 2 O(l) with the addition of ammonia (NH 3) precipitation of metal hydroxides by controlled changes of pH is perhaps the best known and most and, by use of the solubility line of the required metal, to read off the equilibrium metal ion. The aim of this text is to show how the solubility (S) of metal hydroxides, oxidehydroxides, and oxides can be calculated as function of the equilibrium pH of the solutions, taking into account the formation of various cationic and anionic species of metal aqua ions; however excluding all foreign ligands. Metal hydroxides (OH Solubility is Affected by pH. The pH of an aqueous solution can affect the solubility of the solute. By changing the pH of the solution, you can change the charge state of the solute. If the pH of the solution is such that a particular molecule carries no net electric charge, the solute often. solubility of the hydroxides, sulphates and carbonates of the group 2 elements in water This page looks at the solubility in water of the hydroxides, sulphates and carbonates of the Group 2 elements beryllium, magnesium, calcium, strontium and barium. The precipitation of metal hydroxides above a critical pH has long been solubility product of copper hydroxide at 1108 M. It is important to note that the domain titration curve) of copper hydroxide relative to the rate of pH change in the experiment. Answer the following questions using the solubility curve on the right. What salt is least soluble in water at 20 C Ce2(SO4)3. How many grams of Enthalpy of Formation of K011(cr). Equilibrium chemistry software from OLI Systems, Inc. (Morris Plains, NJ) was used to predict the solubility of metal hydroxidesoxides in water as a function of pH. metal ion reacts with the Hydroxide Ion to form an insoluble Metal Hydroxide. Metal Hydroxide (caustic or Lime) Metal Hydroxide Precipitate The solubility curve below shows the. Sodium hydroxide is a popular strong base used in industry. Around 56 of sodium hydroxide produced is used by industry, 25 of which is used in the paper industry. Sodium hydroxide is also used in the manufacture of sodium salts and detergents, pH regulation, and organic synthesis.
Related Images:
- National Building Cost Manuals
- Toyota Dolphin Rv Repair Manuals
- Fall of the star
- I feel so good
- Basic instinc 2
- Heat Transfer Holman Solution Manual 6Th Edition
- Beer Tap into the Art and Science of Brewing
- Justin timberlake 2
- Cowboys alien latino
- Saturday night live s36e07
- Mirages of the mind
- The nutcracker in 3d 2010 hindi
- Marvels agents s h i e l d s02e05
- Big bang theory s06e19 720p
- Fate zero s1
- Download bluestacks hd app player pro setup xp
- Lisbon the walkmen
- Sisterhood episode 4
- Kingdom Hearts Birth by Sleep psp
- March Book One By John Lewis
- Turtle power the definitive history
- Its a girl
- Nani hindi movie
- The Oxford Bible Commentary
- Remember the titans mp4
- Night in paris
- Age of tomorrow 2018
- Tragically hip in between
- Ps3 dark souls ii
- Lost girl s01e09
- Literacy Centers
- Compendio De Patologia De Robbins 7 Edicion Pdf
- Engineering geology ppt download
- The goldbergs 2018 s01
- Quickbooks for Mac
- A mulher invisivel
- The soup 2018 07
- Disney classic volume
- Once upon a time the doctor
- The fate of love
- Mana falta amor
- Clash of titans
- La guerre des rose
- Wiz Khalifa Blacc Hollywood
- Voice kids nl
- Office pro plus 15
- Unit On Wonder By Rj Palacio
- Investment Science By David G Luenberger Answer
- Once upon a time s01e02 720p web dl
- Hansel And Gretel Witch Hunters
- Beverpatroelje
- Florence and the machine ceremonials
- Two girls in love
- Video game high school
- Greatest hits smokie
- Evolution Irfan Yilmaz
- The Art and Technique of Digital Color Correction
- Rabbids invasion season 2
- Walmart Auto Hours Of Operation
- A bite of china s02e03
- Assholism By Xavier Crement Pdf
- Libro rojo amanecer pdf
- Double Dynamite
- Supreme ntm best of
- The cure for ebola
- Renkin sankyuu magical pokaan
- The Universe 3d
- The adventures of spider man
- Jenny Air Compressor Wiring Diagram
- Cherry jul cherry
- Pbs the national parks americas best idea the morn
- Octonauts season 1 download torrent
- A guy named joe
- Naruto shippuden ost
- Shake your tail
- 2018 1080p 3d